1. Formulation development is not trial and error.
- It is a structured scientific process where every decision must be supported by data and statistical justification.
2. The purpose of statistics in pharmaceutical R and D is not complex mathematics.
- Its real role is to support decision-making, demonstrate control, and meet regulatory expectations.
3. Statistics helps answer three fundamental development questions.
- Is the formulation consistent.
- Is it comparable to the reference product.
- Is the manufacturing process scientifically controlled.
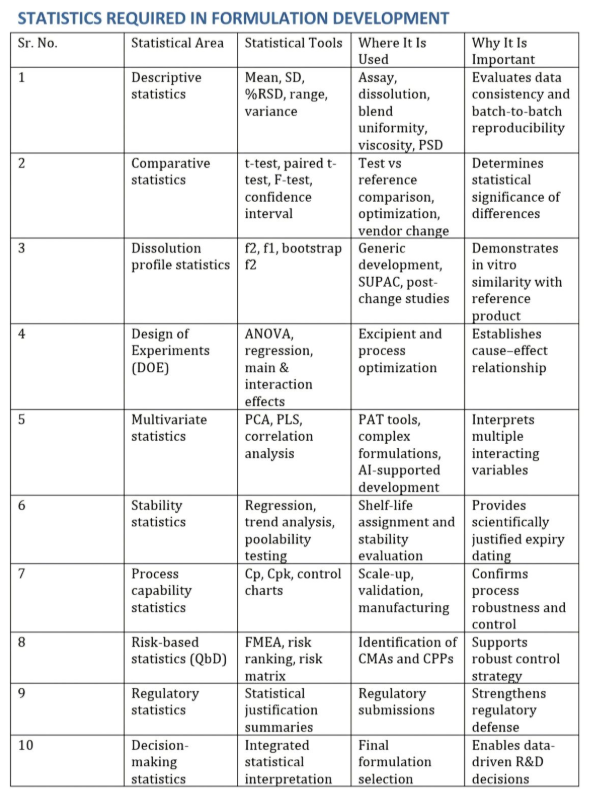
4. Descriptive statistics form the foundation of formulation work.
- Mean, standard deviation, percent RSD, and range are routinely used to evaluate assay, dissolution, blend uniformity, viscosity, particle size, and stability data.
5. Low variability indicates a robust formulation and process.
- High variability highlights formulation weakness or poor process control.
6. Comparative statistics are required whenever changes are made.
- Tools such as t-test, paired t-test, F-test, and confidence interval analysis help determine whether observed differences are statistically significant.
7. Dissolution profile statistics are critical for generic development.
- Similarity factor f2 and difference factor f1 are used to demonstrate in vitro equivalence with the reference product.
8. Design of Experiments transforms development from trial and error into scientific understanding.
- DOE explains how formulation and process variables impact critical quality attributes and answers why a batch worked, not just which batch worked.
9. Multivariate statistics are used when multiple variables act simultaneously.
- Techniques such as PCA and PLS support PAT tools, complex formulations, and AI-enabled development.
10. Stability statistics support shelf-life assignment.
- Regression analysis, trend evaluation, and poolability testing ensure expiry dating is scientifically justified.
11. Process capability statistics indicate manufacturing readiness.
- Cp, Cpk, and control charts demonstrate whether the process can consistently meet specifications.
12. Risk-based statistical tools link development with Quality by Design.
- FMEA and risk ranking help identify critical material attributes and critical process parameters.
13. Regulators do not expect advanced mathematics from formulation scientists.
- They expect logical application of statistics to demonstrate understanding, reproducibility, and control.
14. Statistics does not replace formulation science.
- It strengthens it.
15. In modern pharmaceutical R and D, statistical understanding is no longer optional.
- It is a core competency for every formulation scientist.
Read also: Various Statistical Methods in Pharma | A Key to Data-Driven Decisions
Resource Person: Moinuddin Syed. Ph.D, PMP®
